- Pharmacovigilance systems are an integral part of healthcare policies in many jurisdictions. In the EU, both marketing authorization holders and member countries are responsible for monitoring the safety of authorized drugs. Hence, pharmacovigilance in the EU is a system of shared responsibility and cooperation.
- It is a system and activity contributing to the protection of patients and public by; Preventing harm from adverse reactions in humans, and Promoting the safe and effective use of medical products.
- A pharmacovigilance system is designed to monitor the safety of authorized medicinal products and detect any change to their risk-benefit balance using quality systems that are adequate and effective for the PV performance.
- It is used by an organization, marketing authorization holder and by medicine authorities to fulfill its legal tasks and responsibilities in relation to pharmacovigilance. Quality requirements are those characteristics of a system that are likely to produce the desired outcome, or quality objectives.
- Principles for good pharmacovigilance practices should guide the design of all structures and processes. As well as, the conduct of all tasks and responsibilities.
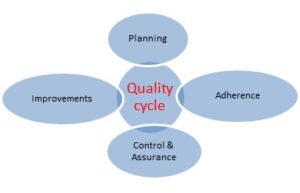
- For the purpose of a systematic approach towards quality in accordance with the quality cycle; managerial staff (i.e. staff with management responsibilities) should be available in any organization responsible for the quality system performance.
Record Management: The organization shall record all pharmacovigilance information and ensure that it is handled and stored so as to allow accurate reporting, interpretation and verification of that information. This shall be put in place for all documents used for pharmacovigilance activities.
- The marketing authorization holder in the Arab Country concerned is responsible for the respective pharmacovigilance tasks and responsibilities in order to assure responsibility and liability for its authorized medicinal products by operating a pharmacovigilance system and shall establish and use a quality system.
- A description of the pharmacovigilance system shall be developed by the applicant for a marketing authorization in the format of a pharmacovigilance system master file (PSMF) and be maintained by the marketing authorization
holder for all authorized medicinal products.
- The applicant or the marketing authorization holder is also responsible for developing and maintaining product specific risk management systems.
Overall PV Responsibilities within each of the Arab Countries: The national medicines authorities in the Arab Countries are responsible for the respective pharmacovigilance tasks and responsibilities in order to ensure that appropriate action can be taken, when necessary.
The national medicines authorities in the Arab Countries are responsible for the respective pharmacovigilance tasks and responsibilities in order to ensure that appropriate action can be taken, when necessary.
Pharmacovigilance Software Marketing / Installation:
- We can serving your organization by hosting with validated Computerized PV database complied with international agreed standards ICH-E2B, to be acquired with Pharmacovigilance compliance with data migration and integration.
- Hence, our licensed national PV software can manage unique use in Pharmacovigilance Safety – easy phV. Validation documents are generated and supplied by us to all customers.
All Applications offer the following features;
- Audit trail – Edition of regulatory reports (Sum Tab,CIOMS,MedWatch,E2B XML.)
- Access to a query module on all fields (Generation Excel files)
- Date Entry “user friendly”
- Access to MedDRA coding with SMQ.